In the quest to unlock effective Alzheimer’s treatment, researchers are turning their attention towards TIM-3 and its critical role in Alzheimer’s research. Recent studies suggest that this immune checkpoint molecule could herald a new approach in tackling neurodegenerative diseases like Alzheimer’s by enhancing the immune system’s ability to clear harmful plaques from the brain. By understanding the mechanisms behind TIM-3 modulation, scientists hope to devise therapies that not only improve cognitive function but also restore memory in patients. Much like its application in cancer treatment, where TIM-3 inhibition allows T cells to mount a stronger attack against tumors, similar strategies may empower microglial cells in the brain to combat the amyloid plaques characteristic of Alzheimer’s. Given the urgency for breakthroughs in Alzheimer’s treatment, this innovative research presents a promising avenue for future immune system therapy that could reshape the landscape of neurodegenerative disease management.
The exploration of TIM-3 in the context of Alzheimer’s disease opens up new discussions around innovative therapeutic strategies for combating this pervasive condition. As researchers investigate the implications of checkpoint molecules on brain immunity, they are uncovering parallels with successful cancer therapies that utilize similar mechanisms. By inhibiting TIM-3, it may be possible to enhance the functionality of microglial cells, the brain’s primary immune defenders, thus enabling them to eliminate toxic amyloid plaques that disrupt cognitive processes. This exciting research not only advances our understanding of neurodegenerative diseases but also highlights potential pathways for developing novel treatments aimed at preserving memory and cognitive health in aging populations. As the field of Alzheimer’s research evolves, integrating these insights could lead to breakthrough therapies that transform the lives of patients and their families.
The TIM-3 Molecule: A New Frontier in Alzheimer’s Research
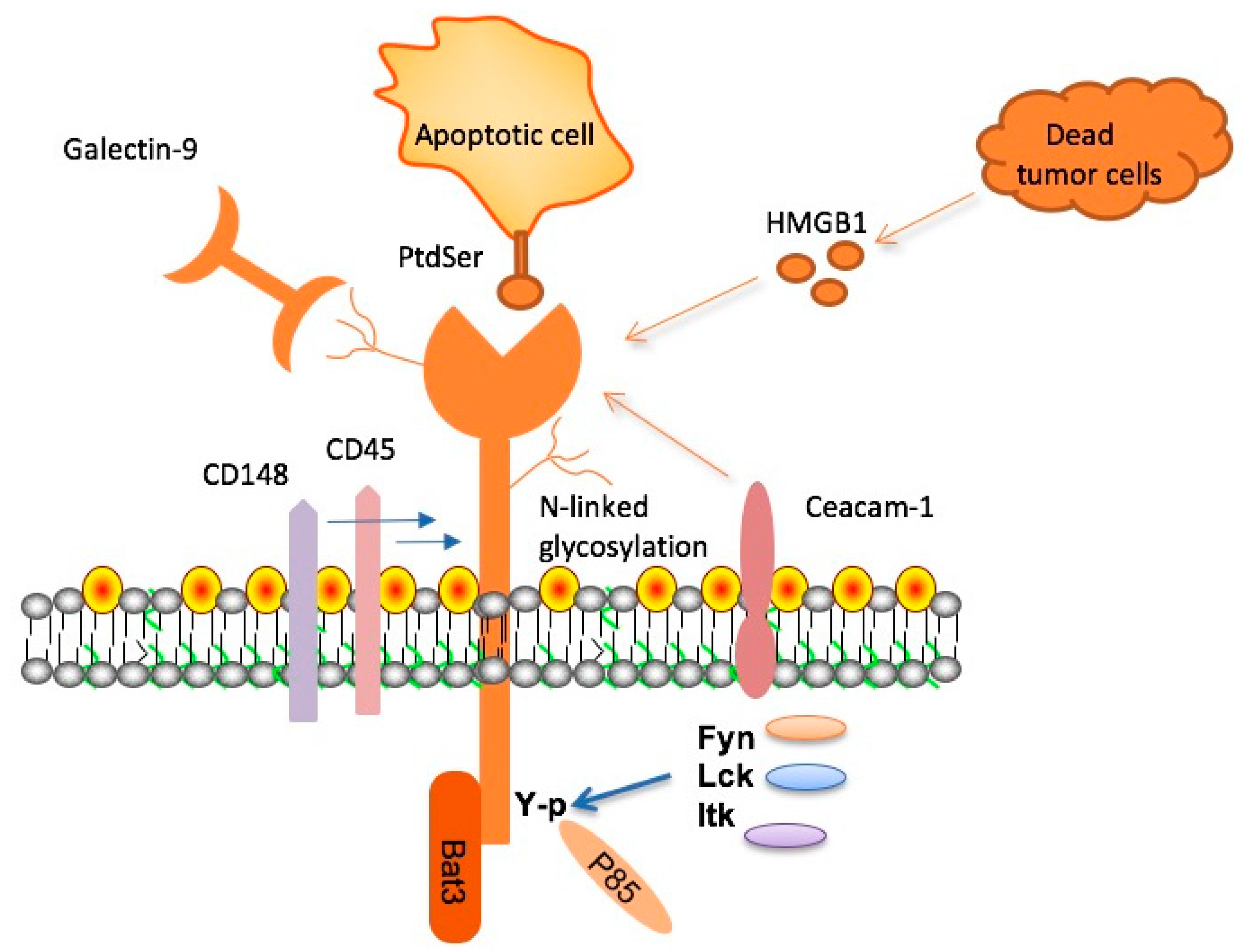
Recent studies have illuminated the TIM-3 molecule’s role in Alzheimer’s disease, revealing its potential as a critical target for treatment. TIM-3 is an immune checkpoint molecule typically involved in regulating T cell activity during immune responses. In the context of Alzheimer’s, however, it inhibits the brain’s immune cells, the microglia, effectively preventing them from clearing amyloid plaques. Research shows that the deletion of TIM-3 can enhance microglial activity, allowing these cells to better interact with and eliminate harmful plaques, ultimately leading to improvements in memory function in mouse models.
This discovery not only underlines the significance of TIM-3 in late-onset Alzheimer’s cases—comprising 90% to 95% of instances—but also highlights its dual role. While TIM-3 protects the body from overactive immune responses in cancers, its overexpression in Alzheimer’s hampers the brain’s ability to manage toxic plaque accumulation. Understanding the genetic basis and expression patterns of TIM-3 may open doors to novel therapeutic strategies that can harness the power of the immune system to combat neurodegenerative diseases effectively.
Exploring Immune System Therapy Strategies for Alzheimer’s Treatment
With the promising results observed in the TIM-3 studies, researchers are enthusiastic about the prospects of immune system therapy as a treatment for Alzheimer’s disease. The principle behind this approach involves breaking the inhibitory effect of TIM-3 on microglia, thereby restoring their ability to clear amyloid plaques. Given the complexities associated with Alzheimer’s pathology, employing immune checkpoint inhibitors, which have seen success in cancer treatment, presents a compelling strategy to invigorate the brain’s immune response against neurodegeneration.
Furthermore, the connection between immune system pathways and Alzheimer’s disease suggests that therapies designed to block TIM-3 might provide more than just symptomatic relief. They could potentially halt or even reverse cognitive decline by addressing the root cause: plaque formation and neuroinflammation. As researchers move forward with clinical trials, the integration of existing cancer treatments into Alzheimer’s research represents a bright intersection of oncology and neurology, with the potential to transform our approach to treating this devastating disease.
Harnessing the body’s immune response in a regulated way can yield significant advancements not only in combating Alzheimer’s but also in enhancing overall understanding of related neurodegenerative conditions. By identifying which patients might benefit most from TIM-3 inhibition, tailored therapies could elevate clinical outcomes dramatically.
The Role of Checkpoint Molecules in Alzheimer’s Disease
Checkpoint molecules traditionally serve to downregulate immune responses, ensuring the body does not attack healthy tissue. However, in diseases such as Alzheimer’s, these molecules can obstruct essential immune functions. As demonstrated with TIM-3, while it plays a protective role against autoimmunity, in the dementia context, its expression hinders microglial cell function. This cellular response can deteriorate the brain’s health by preventing the timely clearance of amyloid beta plaques, indicative of Alzheimer’s progression.
Research highlights that microglia, the brain’s resident immune cells, exhibit altered behavior as they age, including increased TIM-3 expression. This transition to what is termed a homeostatic state leads to an inability to prune away debris effectively, exacerbating Alzheimer’s pathology. Understanding how checkpoint molecules can be modulated could lead to innovative strategies to rejuvenate microglial activity, aligning with the mission to improve cognitive health in Alzheimer’s patients.
The Implications of TIM-3 Deficiency in Alzheimer’s Models
Experimental models of Alzheimer’s disease have provided pivotal insights into the role of TIM-3 deficiency in cognitive performance. By deleting the HAVCR2 gene,which codes for TIM-3, researchers observed an increased clearance of amyloid plaques by microglia. This not only leads to a reduction in plaque burden but also positively impacts memory and navigation abilities in these animal models. Such findings indicate that there is a direct correlation between TIM-3 activity and cognitive decline related to Alzheimer’s.
The implication of these results underscores the necessity for targeted therapies that can modulate TIM-3 levels as a part of a comprehensive strategy to address Alzheimer’s disease. Future studies will likely focus on the translation of these findings into human studies, assessing the potential for similar outcomes in patients with this devastating condition. As the research progresses, the hope is that TIM-3 could emerge as a therapeutic target that helps restore cognitive function by re-engaging the brain’s own immune defenses.
Future Directions for TIM-3 Research in Alzheimer’s Treatment
The ongoing exploration of TIM-3 as a therapeutic target in Alzheimer’s treatment raises exciting possibilities for future research. As scientists delve deeper into the mechanisms by which TIM-3 influences microglial function and plaque formation, they aim to develop specific inhibitors or antibodies. This could pave the way for groundbreaking clinical interventions that enable patients to reclaim cognitive functions. The success of targeting checkpoint molecules in cancer informs this approach, suggesting a new paradigm in combating neurodegenerative diseases.
Future investigations will also look into the potential for combination therapies that integrate TIM-3 inhibitors with existing Alzheimer’s interventions. Such strategies could enhance the overall efficacy of treatment by engaging multiple pathways involved in disease pathology. With significant strides being made in understanding immune modulation in neurodegenerative conditions, research into TIM-3 provides a promising avenue for developing more effective solutions for Alzheimer’s and potentially other related diseases.
TIM-3 and the Intersection of Cancer Treatment and Alzheimer’s Research
The advent of immune checkpoint therapy in cancer treatment has created a ripple effect across various medical research fields, including that of Alzheimer’s disease. The TIM-3 molecule offers a unique intersection between these two disciplines, showcasing how strategies developed for treating malignancies may be adapted to combat neurodegenerative conditions. Researchers are beginning to realize that the same principles that allow tumors to elude immune detection may also contribute to the failure of microglia in clearing toxic plaques in the brain.
As clinical trials advance, the potential to repurpose existing anti-TIM-3 antibodies for Alzheimer’s therapy could represent a significant leap forward. By leveraging the knowledge gained from oncology, Alzheimer’s researchers are better equipped to devise innovative therapeutic interventions that might restore microglial clearance capabilities. The excitement within the scientific community is palpable as breakthrough discoveries continue to bridge the knowledge gap between cancer treatment and neurodegenerative disease management.
Understanding the Genetic Link of TIM-3 to Alzheimer’s
The genetic underpinnings of Alzheimer’s disease are complex, yet the role of TIM-3 has been highlighted as a significant focus in current research. Genome-wide association studies have linked polymorphisms in the HAVCR2 gene, responsible for encoding TIM-3, to late-onset Alzheimer’s morbidity. This connection is crucial because it elucidates the potential inherited risk factors that predispose individuals to amyloid accumulation and cognitive decline.
Recent findings reveal that individuals with certain TIM-3 genetic variations show marked differences in microglial TIM-3 expression. Such insights reinforce the need for personalized medicine approaches in Alzheimer’s treatment. Identifying genetic susceptibility can assist clinicians in tailoring therapeutics that modulate TIM-3, ultimately leading to improved patient outcomes by addressing the specific mechanisms associated with plaque clearance and neurodegeneration.
Potential Challenges in Targeting TIM-3 for Alzheimer’s Therapy
While targeting TIM-3 presents promising avenues for Alzheimer’s therapy, it is not without its challenges. The nuanced role of TIM-3 in both the immune system and brain health complicates the approach, as inhibiting its function may lead to unforeseen consequences, such as increased susceptibility to infections or autoimmunity. Researchers must carefully navigate these challenges as they develop therapies aimed at modulating TIM-3, ensuring that the benefits in plaque clearance do not come at the cost of overall immune function.
Rigorous preclinical testing and clinical trials will be necessary to establish the safety and efficacy of TIM-3 inhibitors in Alzheimer’s disease patients. The excitement surrounding this research is tempered by the reality that transitioning from successful animal models to human applications requires thorough examination. Ultimately, overcoming these barriers will be key to unlocking the potential of TIM-3 as a viable therapeutic target in the fight against Alzheimer’s.
The Promise of Anti-TIM-3 Antibodies in Alzheimer’s Treatment
The exploration of anti-TIM-3 antibodies holds great promise for revolutionizing Alzheimer’s treatment strategies. By blocking the inhibitory effects of TIM-3, these antibodies could significantly enhance the ability of microglia to clear amyloid-beta plaques, potentially improving cognitive abilities for patients suffering from this disease. As existing antibodies are repurposed for this new context, the transition from laboratory findings to therapeutic applications is eagerly anticipated in the scientific community.
As the research progresses, the combination of immunotherapy and traditional approaches may yield a more holistic treatment model for Alzheimer’s disease. The integration of anti-TIM-3 antibodies into Alzheimer’s treatment regimens could redefine patient care and broaden the scope of therapeutic options available. With each advancement, the prospect of leveraging immune checkpoint inhibitors for neurodegenerative conditions continues to create excitement and hope among researchers and patients alike.
Frequently Asked Questions
What is TIM-3 and how does it relate to Alzheimer’s research?
TIM-3, or T-cell immunoglobulin and mucin domain 3, is a checkpoint molecule that plays a key role in regulating the immune response. Recent Alzheimer’s research highlights TIM-3’s involvement in inhibiting microglial cells, the brain’s immune cells, from clearing amyloid plaques. By blocking TIM-3, studies found that microglia can more effectively attack these plaques, potentially improving cognitive function in Alzheimer’s disease.
How does TIM-3 affect microglial function in Alzheimer’s disease?
In Alzheimer’s disease, TIM-3 inhibits microglial cells from engulfing amyloid plaques, which leads to plaque accumulation in the brain. Research indicates that deleting or blocking TIM-3 expression enhances the ability of microglia to clear these plaques, reducing cognitive impairment and improving memory in Alzheimer’s disease models.
Can TIM-3 targeting be considered for Alzheimer’s treatment?
Yes, targeting TIM-3 has potential implications for Alzheimer’s treatment. Researchers are investigating the use of anti-TIM-3 antibodies or small molecules to block TIM-3’s inhibitory effects, enabling microglia to better clear amyloid plaques. This represents a novel approach in Alzheimer’s treatment aimed at enhancing immune function in the brain.
What role do immune system therapy and TIM-3 play in Alzheimer’s research?
Immune system therapy utilizing TIM-3 has emerged as a promising area of Alzheimer’s research. By understanding how TIM-3 regulates microglial response to plaque accumulation, scientists can develop therapies designed to boost the immune system’s ability to combat Alzheimer’s. Such therapies may resemble successful strategies already used in cancer treatment by repurposing TIM-3 modulation techniques.
What were the findings of the recent study involving TIM-3 in Alzheimer’s mice models?
The study found that genetically deleting the TIM-3 gene in Alzheimer’s mice led to enhanced plaque clearance by microglia, resulting in improved cognitive functions. Mice without TIM-3 exhibited better memory retention and navigation abilities, indicating that targeting this checkpoint molecule could potentially reverse some cognitive decline associated with Alzheimer’s.
Why is there a connection between TIM-3 and late-onset Alzheimer’s disease?
TIM-3 has been linked to late-onset Alzheimer’s disease through genome-wide association studies, identifying it as a genetic risk factor. The polymorphism in the TIM-3 gene correlates with increased expression in microglia from Alzheimer’s patients, highlighting its role in inhibiting the clearance of amyloid plaques, which are characteristic of the disease.
How could TIM-3 therapies impact future Alzheimer’s treatment options?
Therapeutic interventions that target TIM-3 may enhance current Alzheimer’s treatment paradigms by providing a novel mechanism for promoting plaque clearance. This strategy could bypass limitations seen in traditional amyloid-targeting therapies and offer a more effective means of addressing the underlying neurodegeneration seen in Alzheimer’s disease.
What are the next steps in TIM-3 related Alzheimer’s research?
Future steps involve testing human anti-TIM-3 antibodies in mouse models with the human TIM-3 gene introduced. This research aims to determine if these antibodies can effectively inhibit plaque development and cognitive decline in Alzheimer’s disease, progressing towards potential clinical applications.
| Key Point | Details |
|---|---|
| Research Background | Study published in Nature shows TIM-3 as a potential target for Alzheimer’s treatment. |
| Role of TIM-3 | TIM-3 is an inhibitory molecule linked to late-onset Alzheimer’s, preventing microglia from attacking amyloid plaques. |
| Microglial Function | Microglia are brain immune cells that prune synapses but can become dysfunctional with aging. |
| Effects of TIM-3 Deletion | Deleting TIM-3 in mice enhances plaque clearance and improves cognitive behavior. |
| Therapeutic Potential | Future therapies may involve anti-TIM-3 antibodies to promote plaque removal in Alzheimer’s patients. |
| Collaboration and Duration | Research took five years, with a collaboration involving multiple labs. |
Summary
TIM-3 and Alzheimer’s Research are at the forefront of a revolutionary approach that may change the trajectory of Alzheimer’s treatment. The recent study led by Vijay Kuchroo and his team suggests that targeting TIM-3, an immune checkpoint molecule, could enhance how microglia—brain immune cells—function in clearing amyloid plaques associated with Alzheimer’s disease. This innovative research opens up promising pathways for new therapies, emphasizing the potential of repurposing existing cancer treatments for Alzheimer’s, which could significantly improve cognitive function in affected patients.