Medical research funding is essential for advancing healthcare and ensuring patient safety in research studies. As federal financial support wanes, driven by factors such as NIH funding cuts, the critical oversight expected from Institutional Review Boards (IRBs) is jeopardized. The impact of research funding on ethical practices cannot be overstated, as these funds directly influence the ability to conduct thorough reviews, maintain high standards of patient safety, and uphold research ethics. Without adequate financial resources, the integrity of clinical trials and the welfare of participants become increasingly vulnerable to compromise. In this crucial moment, it is paramount to recognize the significant effect that research funding has on our healthcare advancements and the ethical obligations we owe to every individual involved in medical studies.
Investment in biomedical research, often referred to as medical research funding, plays a pivotal role in enhancing patient safety and supporting ethical standards in clinical studies. With the ongoing challenges posed by reduced federal grants and the complexities of IRB oversight, the research landscape faces significant hurdles. The flow of financial resources directly correlates with the effectiveness of oversight measures designed to protect study participants and ensure compliance with research ethics. As funding sources dwindle, the ability to effectively monitor and manage research initiatives diminishes, posing risks to both public trust and the advancement of medical knowledge. Understanding how financial support underpins every aspect of healthcare-related research is vital for fostering a safe environment for all participants.
The Importance of Medical Research Funding
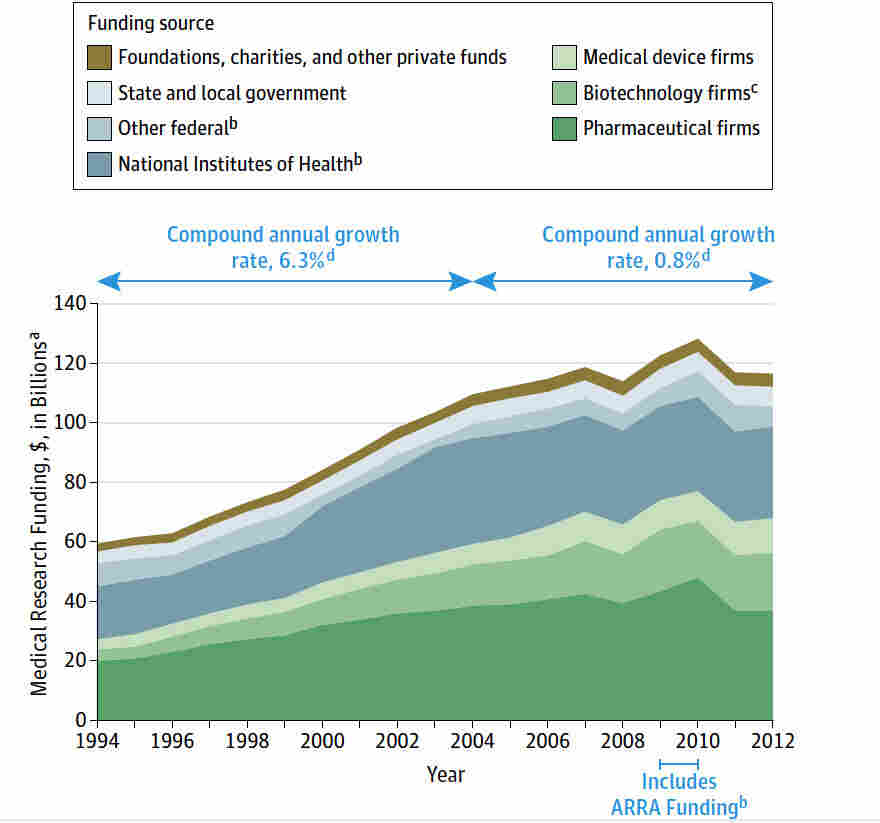
Medical research funding plays a critical role in developing new treatments and advancing healthcare. When governments or organizations allocate funds for research, they are essentially investing in the future of medicine and public health. This financial support allows researchers to explore innovative concepts and conduct thorough studies necessary for transforming theoretical medical ideas into practical applications. In the absence of adequate funding, projects may stall, leading to missed opportunities for breakthroughs that could save lives.
Furthermore, funding is pivotal for maintaining ethical standards and safety measures in medical research. With substantial investments, researchers can afford the resources necessary to implement human subject protections, proper oversight, and rigorous testing protocols. Without such financial backing, there is a risk of compromising patient safety and undermining the integrity of the research process. The recent cuts to NIH funding exemplify the potential disruptions that can arise, which threaten both ongoing studies and participant welfare.
Frequently Asked Questions
What impact do NIH funding cuts have on patient safety in medical research?
NIH funding cuts can severely undermine patient safety by disrupting essential oversight mechanisms in medical research. With reduced funding, Institutional Review Boards (IRBs) may struggle to ensure compliance with ethical standards and regulatory requirements, potentially leading to inadequate participant protection in research studies.
How does the IRB oversight process contribute to the impact of research funding on patient safety?
IRB oversight is crucial for protecting research participants, as it ensures that all studies comply with ethical guidelines and federal regulations. When research funding is insufficient, IRB resources can be compromised, leading to rushed reviews and potentially related safety issues for patients involved in clinical trials.
In what ways do funding cuts affect research ethics in medical studies?
Funding cuts can lead to compromised research ethics, as institutions may prioritize project completion over thorough ethical review and oversight. This compromise can jeopardize informed consent processes and participant welfare, diminishing the ethical standards upheld in medical research.
How are patient rights protected through NIH-funded medical research?
NIH-funded medical research incorporates rigorous review processes where IRBs assess protocols to ensure the protection of patient rights. Proper funding allows these boards to function effectively, safeguarding participants’ rights and welfare during clinical trials.
What role does research funding play in the effectiveness of patient safety measures in clinical trials?
Research funding is vital for implementing comprehensive patient safety measures. Adequate funding enables institutions to maintain robust IRB oversight, continuous monitoring, and training, ultimately supporting the ethical treatment of research subjects and enhancing overall patient safety.
Can NIH funding cuts impact the collaborative research environment?
Yes, NIH funding cuts can significantly affect collaborative research environments by leading to halted or delayed multi-site studies. Such interruptions make it difficult for research teams to share data and uphold patient safety standards, as essential collaborative measures may no longer be viable.
How does the history of medical research inform current standards for patient safety in funded studies?
Historical abuses in medical research, such as the Tuskegee syphilis study, have shaped current patient safety standards. These past events underscore the need for accountable funding and regulatory oversight, ensuring that modern NIH-funded studies prioritize ethical considerations and participant protection.
What steps can be taken to mitigate the effects of research funding cuts on patient safety?
To mitigate the effects of research funding cuts on patient safety, institutions can advocate for increased federal funding, raise awareness about the importance of research oversight, and foster community partnerships that emphasize transparent practices and participant protection in medical research.
| Key Point | Details |
|---|---|
| Impact of Funding Cuts | $2 billion in frozen federal research grants to Harvard disrupts oversight of patient safety in medical research. |
| Role of IRBs | Institutional Review Boards (IRBs) ensure the rights, welfare, and safety of research participants through rigorous proposal reviews. |
| NIH’s Policy on IRB Oversight | The NIH mandates collaborative research to be overseen by a single IRB, streamlining oversight responsibilities across multiple sites. |
| Historical Context | Significant ethical breaches in history highlight the importance of oversight in protecting research participants. |
| Consequences of Disruption | Halting ongoing studies risks participant safety and reinforces public distrust in medical research. |
| Continuation of Research | Support from Harvard Medical School aims to maintain collaborative research efforts despite funding challenges. |
Summary
Medical research funding is critical for ensuring the safety and ethical oversight of clinical studies involving human participants. The recent freeze of over $2 billion in federal research grants to Harvard has severely hindered initiatives designed to protect the rights and welfare of patients involved in medical research. This disruption not only affects ongoing studies but also jeopardizes public trust in the research community. As the reliance on institutional review boards (IRBs) grows, it’s essential to advocate for continued funding to safeguard participants and enhance the ethical standards in medical research.